Comprehensive Guide to Peptide Reconstitution and Preparation for Research Applications
November 14th, 2025 7:40 AM
By: Newsworthy Staff
This technical guide provides essential protocols for accurate peptide reconstitution, concentration calculations, and storage methods that are critical for ensuring experimental reproducibility and reliability in scientific research.
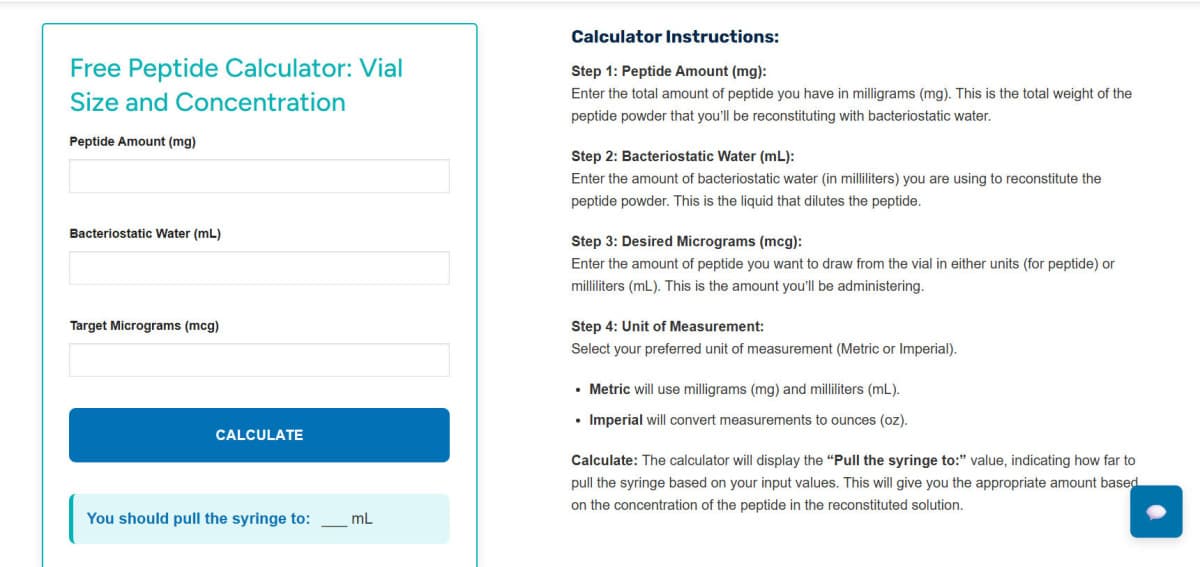
Precise calculation and reconstitution of peptide vials are critical for ensuring reproducibility in research workflows. This comprehensive guide outlines the process for determining concentration of stock solutions, executing unit conversions, reconstituting peptides in sterile environments, preparing working dilutions, and addressing common issues related to solubility or aggregation. The techniques described are fundamental to maintaining experimental integrity across various research applications.
Peptide concentration calculation follows the standard formula where concentration in mg/mL equals peptide mass in mg divided by diluent volume in mL. To convert this to micrograms per milliliter, multiply mg/mL by 1000. Molarity can also be calculated based on molecular weight using the formula: Molarity (M) equals mg/mL divided by molecular weight in g/mol multiplied by 1000. Understanding unit conversions is crucial, with 1 mg equaling 1000 mcg, and for U-100 insulin syringes, 1 mL equaling 100 IU. This enables conversion of target microgram amounts into accurate draw volumes.
For practical application, if a stock solution has concentration of 5 mg/mL, it equates to 5000 mcg/mL. To find volume required for 250 mcg, the calculation would be 250 mcg divided by 5000 mcg/mL equals 0.05 mL. These calculations ensure precise experimental measurements and consistent preparation of stock solutions. Additional resources and technical specifications are available at https://lotilabs.com for researchers requiring detailed protocols.
Reconstitution involves dissolving lyophilized peptides in appropriate diluent while maintaining sterile conditions. Common diluent options include bacteriostatic water with preservative suitable for multi-use vials, sterile water ideal for single-use aliquots, DMSO effective for dissolving hydrophobic peptides, and low percent acid to enhance solubility of charged peptides. The step-by-step reconstitution process requires setting up clean workspace with syringes, diluent, labels, and personal protective equipment.
The reconstitution procedure involves disinfecting vial septum using alcohol swab, drawing calculated volume of diluent into sterile syringe, injecting slowly along vial wall to minimize foaming, and gently swirling or flicking vial until peptide is fully dissolved. If dissolution remains incomplete, researchers should allow for equilibration, use brief sonication, or add minimal amount of co-solvent. Proper labeling of vials with concentration, solvent, date, and any modifications is essential for tracking and reproducibility.
Storage guidelines specify that lyophilized peptides should be stored in cold, dry environment at -20°C for short-term and -80°C for long-term storage, protected from light and moisture. Reconstituted peptides should be refrigerated for short-term use or frozen as aliquots at -20°C or -80°C for prolonged periods, with limited freeze-thaw cycles. Troubleshooting solubility and aggregation issues involves starting with gentle swirling and flicking, allowing time for equilibration, employing brief sonication when necessary, and cautiously adding small amounts of DMSO or low percent acid for stubborn peptides.
Preventive strategies include proper solvent selection, slow addition to buffers, maintaining suitable pH and ionic strength, aliquoting to minimize freeze-thaw cycles, and avoiding repeated exposure to room temperature. Key considerations emphasize double-checking all calculations and unit conversions, choosing appropriate syringe types for small-volume measurements to reduce relative error, documenting every step including solvents and adjustments, ensuring sterile handling to prevent contamination, and keeping track of stability with clear labeling of all aliquots.
Source Statement
This news article relied primarily on a press release disributed by Press Services. You can read the source press release here,
