Lorati Unveils Nano-Grade Eye Drops for Age-Related Macular Degeneration Treatment
November 19th, 2024 8:00 AM
By: Newsworthy Staff
Lorati Company Limited has developed innovative nano-grade eye drops that show promise in reversing age-related macular degeneration, potentially offering new hope for millions affected by this vision-threatening condition.
In a significant development for ophthalmology, Lorati Company Limited has announced a revolutionary treatment for age-related macular degeneration (AMD) using nano-grade eye drops. This breakthrough has the potential to transform the lives of millions suffering from both dry and wet forms of AMD, a leading cause of vision loss among older adults worldwide.
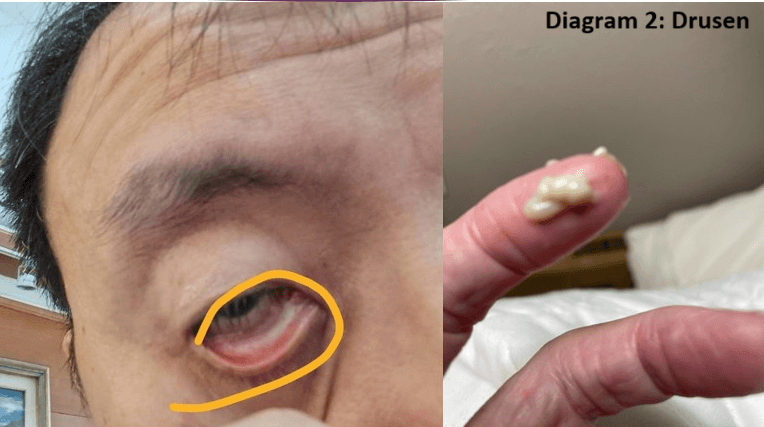
David Lo, CEO of Lorati, revealed that their nano-grade eye drops have successfully reversed AMD in numerous patients, including those previously considered legally blind due to conditions such as drusenoid pigment epithelial detachment (PED). The company's seven-year journey has led to remarkable outcomes, restoring vision and improving the quality of life for dozens of AMD patients.
The innovative eye drops work by simultaneously addressing multiple aspects of AMD pathology. They clear drusen and lipofuscin accumulations while promoting the regeneration of photoreceptor cells, which are critical for vision. Mr. Lo explained that within just one hour of application, the eye drops facilitate the excretion of drusen through the retinal pigment epithelium (RPE), Bruch's membrane, and choriocapillaris complex to the outer conjunctiva. Continued use of the drops leads to gradual vision restoration, offering hope for those who have long struggled with AMD-related vision impairment.
What sets Lorati's treatment apart is its composition. The eye drops are based on montmorillonite, described by the company as an extract of "God's clay." This nano-grade mineral water is reported to be completely safe for human use, contrasting with organic-based eye drops that often come with side effects. The treatment's safety profile could make it an attractive option for patients and healthcare providers seeking effective AMD management with minimal risks.
The implications of this development are far-reaching. AMD affects millions of people globally, with its prevalence expected to increase as populations age. Current treatments for wet AMD, such as regular intravitreal injections, can be invasive and burdensome for patients. For dry AMD, treatment options have been limited. Lorati's eye drops could potentially offer a non-invasive, more accessible treatment modality for both forms of the disease.
However, it's important to note that while the treatment shows promise, it is not a cure. AMD is described as a metabolic syndrome, and the eye drops are part of an ongoing management strategy. A full course of treatment requires 60 ml of the nano-grade mineral water, divided into six 10 ml bottles. Patients are advised to apply the drops up to eight times daily for optimal results, with severe cases potentially requiring four to six courses of treatment.
This innovation could significantly impact the field of ophthalmology and geriatric care. If further validated through rigorous clinical trials and regulatory approvals, it could reduce the economic burden of AMD treatment on healthcare systems and improve the independence and quality of life for millions of older adults worldwide. The potential for a safe, effective, and non-invasive treatment option may also encourage earlier intervention and better management of AMD, potentially slowing or preventing severe vision loss in affected individuals.
As the global population ages and the incidence of AMD rises, Lorati's nano-grade eye drops represent a beacon of hope in the fight against vision loss. While more research and clinical data will be necessary to fully establish the treatment's efficacy and long-term safety, this announcement marks a significant step forward in the quest to combat age-related macular degeneration and preserve vision for older adults around the world.
Source Statement
This news article relied primarily on a press release disributed by 24-7 Press Release. You can read the source press release here,
