Pacylex Advances Revolutionary Cancer Treatment with N-myristoyltransferase Inhibitors
February 3rd, 2025 5:00 AM
By: Newsworthy Staff
Clinical-stage pharmaceutical company Pacylex is making significant strides in cancer treatment through N-myristoyltransferase inhibitors, presenting their progress at major industry conferences. The development represents a potential breakthrough in treating both blood cancers and solid tumors.
Pacylex Pharmaceuticals, a leader in developing N-myristoyltransferase inhibitors (NMTis), is advancing a novel approach to cancer treatment that could transform how both blood cancers and solid tumors are treated. The company's progress will be showcased at three major industry conferences this February, highlighting significant developments in their clinical programs.
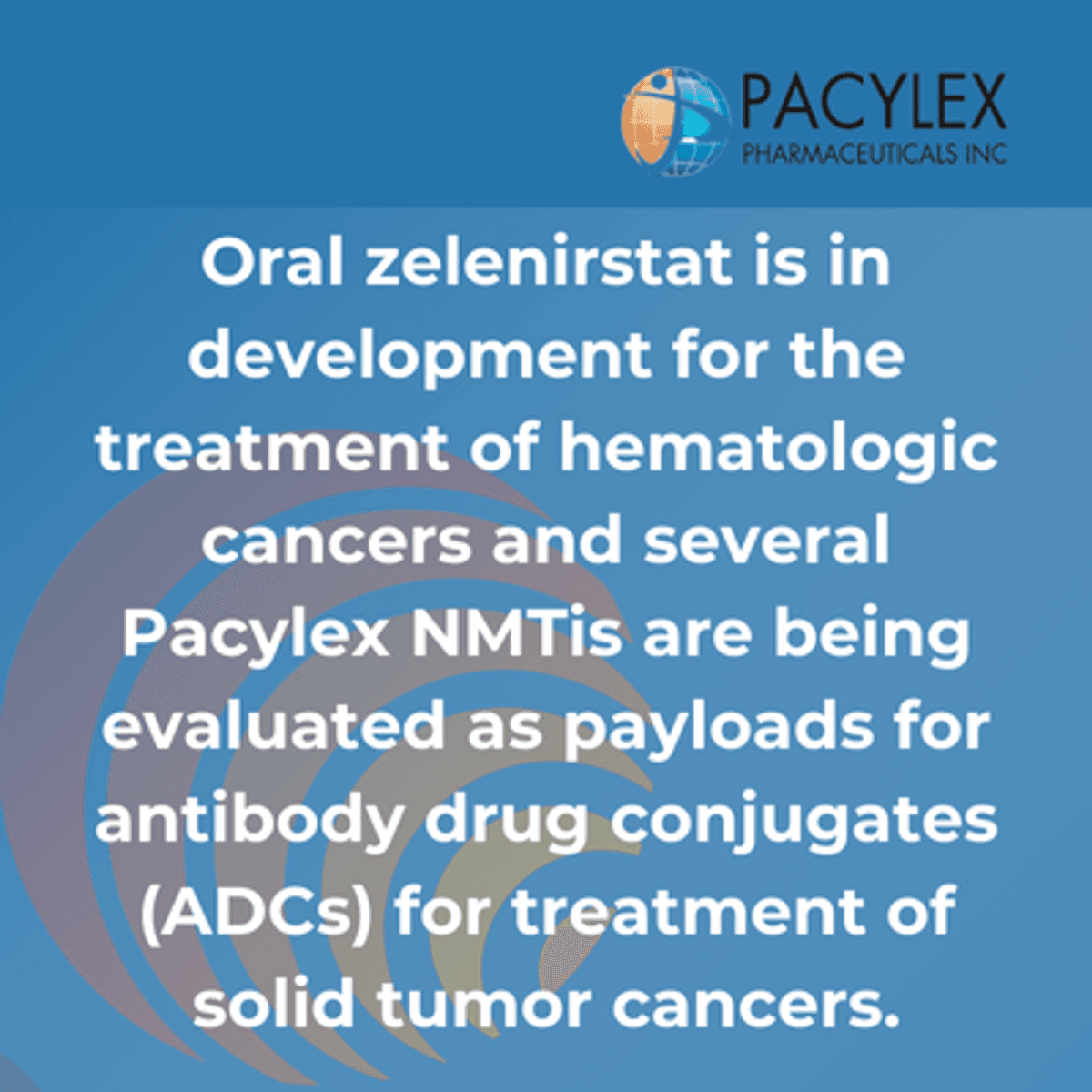
The company's lead drug, oral zelenirstat, represents the first clinically validated NMTi, receiving both Orphan Drug Designation and Fast Track Designation from the FDA for acute myeloid leukemia (AML). This designation underscores the treatment's potential significance in addressing urgent medical needs in blood cancer therapy.
The drug's development carries particular significance due to its dual potential: as an oral treatment for blood cancers and as a payload for antibody drug conjugates (ADCs) in treating solid tumors. In clinical studies, zelenirstat has demonstrated an acceptable safety profile and shown early signs of efficacy, with pharmacokinetics supporting once-daily dosing.
What makes this development particularly noteworthy is the mechanism of action. NMTis work by interfering with multiple pathways essential to cancer cell survival and proliferation, including receptor tyrosine kinases, Wnt pathway, and various signaling pathways crucial for cancer growth. This multi-targeted approach could potentially overcome common resistance mechanisms that limit current cancer treatments.
The company's extensive portfolio of 503 small molecule NMTis, including 28 high-potency compounds, positions them to potentially revolutionize cancer treatment approaches. The technology's versatility in targeting both blood cancers and solid tumors through different delivery methods represents a significant advancement in cancer therapy development.
These developments come at a crucial time in cancer treatment evolution, as researchers seek more effective and targeted therapies. The company's progress, particularly in developing zelenirstat as both an oral medication and ADC payload, could provide new options for patients who have exhausted conventional treatments.
The U.S. Department of Defense's support for initial clinical investigations of zelenirstat in AML patients further validates the potential impact of this treatment approach. This backing, combined with the FDA's special designations, suggests recognition of the technology's potential to address significant unmet medical needs in cancer treatment.
Source Statement
This news article relied primarily on a press release disributed by Reportable. You can read the source press release here,
